Example Assessment¶
After installing PyGauss you should be able to open this IPython Notebook from; https://github.com/chrisjsewell/PyGauss/blob/master/Example_Assessment.ipynb, and run the following...
from IPython.display import display, Image
%matplotlib inline
import pygauss as pg
print 'pygauss version: {}'.format(pg.__version__)
pygauss version: 0.6.0
The test folder has a number of example Gaussian outputs to play around with.
folder = pg.get_test_folder()
len(folder.list_files())
33
Note: the folder object will act identical whether using a local path or one on a server over ssh (using paramiko):
folder = pg.Folder('/path/to/folder',
ssh_server='login.server.com',
ssh_username='username')
Single Molecule Analysis¶
A molecule can be created containg data about the inital geometry, optimisation process and analysis of the final configuration.
mol = pg.molecule.Molecule(folder_obj=folder,
init_fname='CJS1_emim-cl_B_init.com',
opt_fname=['CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_opt-modredundant_difrz.log',
'CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_opt-modredundant_difrz_err.log',
'CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_opt-modredundant_unfrz.log'],
freq_fname='CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_freq_unfrz.log',
nbo_fname='CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_pop-nbo-full-_unfrz.log',
atom_groups={'emim':range(20), 'cl':[20]},
alignto=[3,2,1])
Geometric Analysis¶
Molecules can be viewed statically or interactively.
#mol.show_initial(active=True)
vdw = mol.show_initial(represent='vdw', rotations=[[0,0,90], [-90, 90, 0]])
ball_stick = mol.show_optimisation(represent='ball_stick', rotations=[[0,0,90], [-90, 90, 0]])
display(vdw, ball_stick)
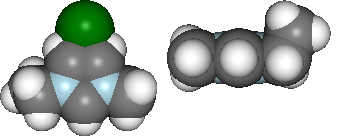
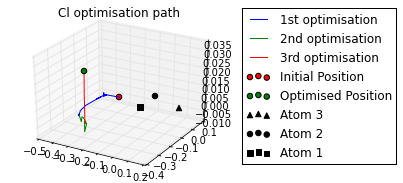
print 'Cl optimised polar coords from aromatic ring : ({0}, {1},{2})'.format(
*[round(i, 2) for i in mol.calc_polar_coords_from_plane(20,3,2,1)])
ax = mol.plot_opt_trajectory(20, [3,2,1])
ax.set_title('Cl optimisation path')
ax.get_figure().set_size_inches(4, 3)
Cl optimised polar coords from aromatic ring : (0.11, -116.42,-170.06)
Energetics and Frequency Analysis¶
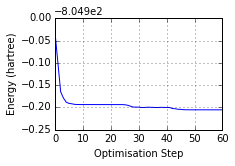
print('Optimised? {0}, Conformer? {1}, Energy = {2} a.u.'.format(
mol.is_optimised(), mol.is_conformer(),
round(mol.get_opt_energy(units='hartree'),3)))
ax = mol.plot_opt_energy(units='hartree')
ax.get_figure().set_size_inches(3, 2)
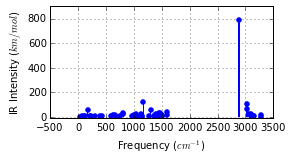
ax = mol.plot_freq_analysis()
ax.get_figure().set_size_inches(4, 2)
Optimised? True, Conformer? True, Energy = -805.105 a.u.
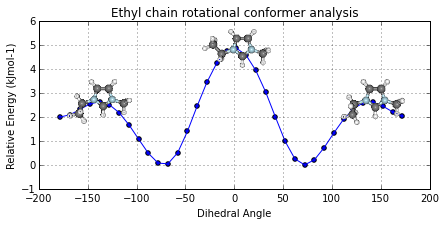
Potential Energy Scan analysis of geometric conformers...
mol2 = pg.molecule.Molecule(folder_obj=folder, alignto=[3,2,1],
pes_fname=['CJS_emim_6311_plus_d3_scan.log',
'CJS_emim_6311_plus_d3_scan_bck.log'])
ax, data = mol2.plot_pes_scans([1,4,9,10], rotation=[0,0,90], img_pos='local_maxs', zoom=0.5)
ax.set_title('Ethyl chain rotational conformer analysis')
ax.get_figure().set_size_inches(7, 3)
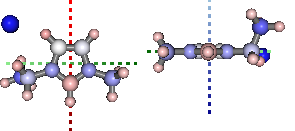
Partial Charge Analysis¶
using Natural Bond Orbital (NBO) analysis
print '+ve charge centre polar coords from aromatic ring: ({0} {1},{2})'.format(
*[round(i, 2) for i in mol.calc_nbo_charge_center(3, 2, 1)])
display(mol.show_nbo_charges(represent='ball_stick', axis_length=0.4,
rotations=[[0,0,90], [-90, 90, 0]]))
+ve charge centre polar coords from aromatic ring: (0.02 -51.77,-33.15)
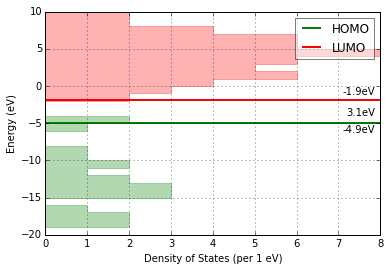
Density of States Analysis¶
print 'Number of Orbitals: {}'.format(mol.get_orbital_count())
homo, lumo = mol.get_orbital_homo_lumo()
homoe, lumoe = mol.get_orbital_energies([homo, lumo])
print 'HOMO at {} eV'.format(homoe)
print 'LUMO at {} eV'.format(lumoe)
Number of Orbitals: 272
HOMO at -4.91492036773 eV
LUMO at -1.85989816817 eV
ax = mol.plot_dos(per_energy=1, lbound=-20, ubound=10, legend_size=12)
Bonding Analysis¶
Using Second Order Perturbation Theory.
print 'H inter-bond energy = {} kJmol-1'.format(
mol.calc_hbond_energy(eunits='kJmol-1', atom_groups=['emim', 'cl']))
print 'Other inter-bond energy = {} kJmol-1'.format(
mol.calc_sopt_energy(eunits='kJmol-1', no_hbonds=True, atom_groups=['emim', 'cl']))
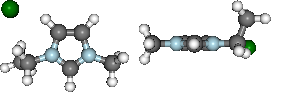
display(mol.show_sopt_bonds(min_energy=1, eunits='kJmol-1',
atom_groups=['emim', 'cl'],
no_hbonds=True,
rotations=[[0, 0, 90]]))
display(mol.show_hbond_analysis(cutoff_energy=5.,alpha=0.6,
atom_groups=['emim', 'cl'],
rotations=[[0, 0, 90], [90, 0, 0]]))
H inter-bond energy = 111.7128 kJmol-1
Other inter-bond energy = 11.00392 kJmol-1
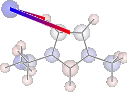
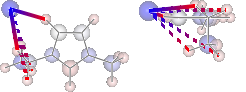
Multiple Computations Analysis¶
Multiple computations, for instance of different starting conformations, can be grouped into an Analysis class and anlaysed collectively.
analysis = pg.Analysis(folder_obj=folder)
errors = analysis.add_runs(headers=['Cation', 'Anion', 'Initial'],
values=[['emim'], ['cl'],
['B', 'BE', 'BM', 'F', 'FE']],
init_pattern='*{0}-{1}_{2}_init.com',
opt_pattern='*{0}-{1}_{2}_6-311+g-d-p-_gd3bj_opt*unfrz.log',
freq_pattern='*{0}-{1}_{2}_6-311+g-d-p-_gd3bj_freq*.log',
nbo_pattern='*{0}-{1}_{2}_6-311+g-d-p-_gd3bj_pop-nbo-full-*.log',
alignto=[3,2,1], atom_groups={'emim':range(1,20), 'cl':[20]},
ipython_print=True)
Reading data 5 of 5
Molecular Comparison¶
fig, caption = analysis.plot_mol_images(mtype='optimised', max_cols=3,
info_columns=['Cation', 'Anion', 'Initial'],
rotations=[[0,0,90]])
print caption
(A) emim, cl, B, (B) emim, cl, BE, (C) emim, cl, BM, (D) emim, cl, F, (E) emim, cl, FE
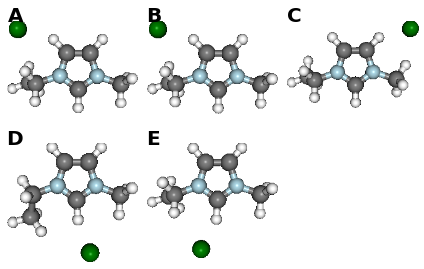
Data Comparison¶
fig, caption = analysis.plot_mol_graphs(gtype='dos', max_cols=3,
lbound=-20, ubound=10, legend_size=0,
band_gap_value=False,
info_columns=['Cation', 'Anion', 'Initial'])
print caption
(A) emim, cl, B, (B) emim, cl, BE, (C) emim, cl, BM, (D) emim, cl, F, (E) emim, cl, FE
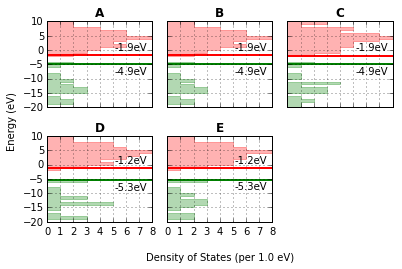
The methods mentioned for indivdiual molecules can be applied to all or a subset of these computations.
analysis.add_mol_property_subset('Opt', 'is_optimised', rows=[2,3])
analysis.add_mol_property('Energy (au)', 'get_opt_energy', units='hartree')
analysis.add_mol_property('Cation chain, $\\psi$', 'calc_dihedral_angle', [1, 4, 9, 10])
analysis.add_mol_property('Cation Charge', 'calc_nbo_charge', 'emim')
analysis.add_mol_property('Anion Charge', 'calc_nbo_charge', 'cl')
analysis.add_mol_property(['Anion-Cation, $r$', 'Anion-Cation, $\\theta$', 'Anion-Cation, $\\phi$'],
'calc_polar_coords_from_plane', 3, 2, 1, 20)
analysis.add_mol_property('Anion-Cation h-bond', 'calc_hbond_energy',
eunits='kJmol-1', atom_groups=['emim', 'cl'])
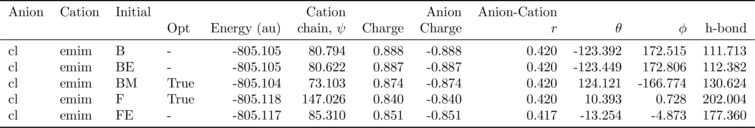
analysis.get_table(row_index=['Anion', 'Cation', 'Initial'],
column_index=['Cation', 'Anion', 'Anion-Cation'])
| Cation | Anion | Anion-Cation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Opt | Energy (au) | chain, $\psi$ | Charge | Charge | $r$ | $\theta$ | $\phi$ | h-bond | |||
| Anion | Cation | Initial | |||||||||
| cl | emim | B | NaN | -805.105 | 80.794 | 0.888 | -0.888 | 0.420 | -123.392 | 172.515 | 111.713 |
| BE | NaN | -805.105 | 80.622 | 0.887 | -0.887 | 0.420 | -123.449 | 172.806 | 112.382 | ||
| BM | True | -805.104 | 73.103 | 0.874 | -0.874 | 0.420 | 124.121 | -166.774 | 130.624 | ||
| F | True | -805.118 | 147.026 | 0.840 | -0.840 | 0.420 | 10.393 | 0.728 | 202.004 | ||
| FE | NaN | -805.117 | 85.310 | 0.851 | -0.851 | 0.417 | -13.254 | -4.873 | 177.360 | ||
There is also an option (requiring pdflatex and ghostscript+imagemagik) to output the tables as a latex formatted image.
analysis.get_table(row_index=['Anion', 'Cation', 'Initial'],
column_index=['Cation', 'Anion', 'Anion-Cation'],
as_image=True, font_size=12)
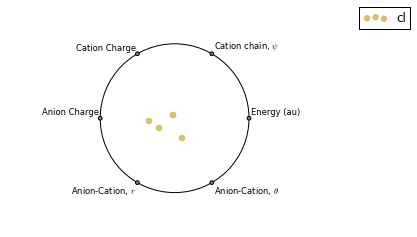
Multi-Variate Analysis¶
RadViz is a way of visualizing multi-variate data.
ax = analysis.plot_radviz_comparison('Anion', columns=range(4, 10))
The KMeans algorithm clusters data by trying to separate samples into n groups of equal variance.
pg.utils.imgplot_kmean_groups(
analysis, 'Anion', 'cl', 4, range(4, 10),
output=['Initial'], mtype='optimised',
rotations=[[0, 0, 90], [-90, 90, 0]],
axis_length=0.3)
(A) BM
(A) B, (B) BE
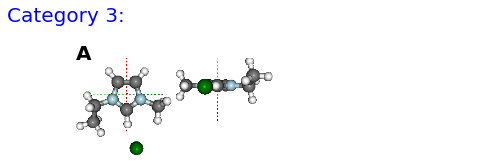
(A) F
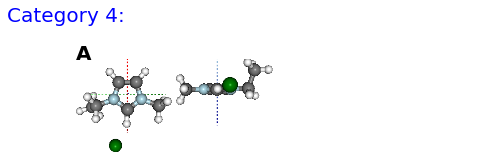
(A) FE
Documentation (MS Word)¶
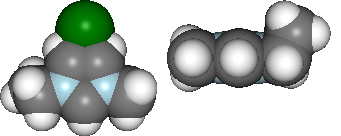
After analysing the computations, it would be reasonable to want to document some of our findings. This can be achieved by outputting individual figure or table images via the folder object.
file_path = folder.save_ipyimg(vdw, 'image_of_molecule')
Image(file_path)
But you may also want to produce a more full record of your analysis, and this is where python-docx steps in. Building on this package the pygauss MSDocument class can produce a full document of your analysis.
import matplotlib.pyplot as plt
d = pg.MSDocument()
d.add_heading('A Pygauss Example Assessment', level=0)
d.add_docstring("""
# Introduction
We have looked at the following aspects
of [EMIM]^{+}[Cl]^{-} (C_{6}H_{11}ClN_{2});
- Geometric conformers
- Electronic structure
# Geometric Conformers
""")
fig, caption = analysis.plot_mol_images(max_cols=2,
rotations=[[90,0,0], [0,0,90]],
info_columns=['Anion', 'Cation', 'Initial'])
d.add_mpl(fig, dpi=96, height=9, caption=caption)
plt.close()
d.add_paragraph()
df = analysis.get_table(
columns=['Anion Charge', 'Cation Charge'],
row_index=['Anion', 'Cation', 'Initial'])
d.add_dataframe(df, incl_indx=True, style='Medium Shading 1 Accent 1',
caption='Analysis of Conformer Charge')
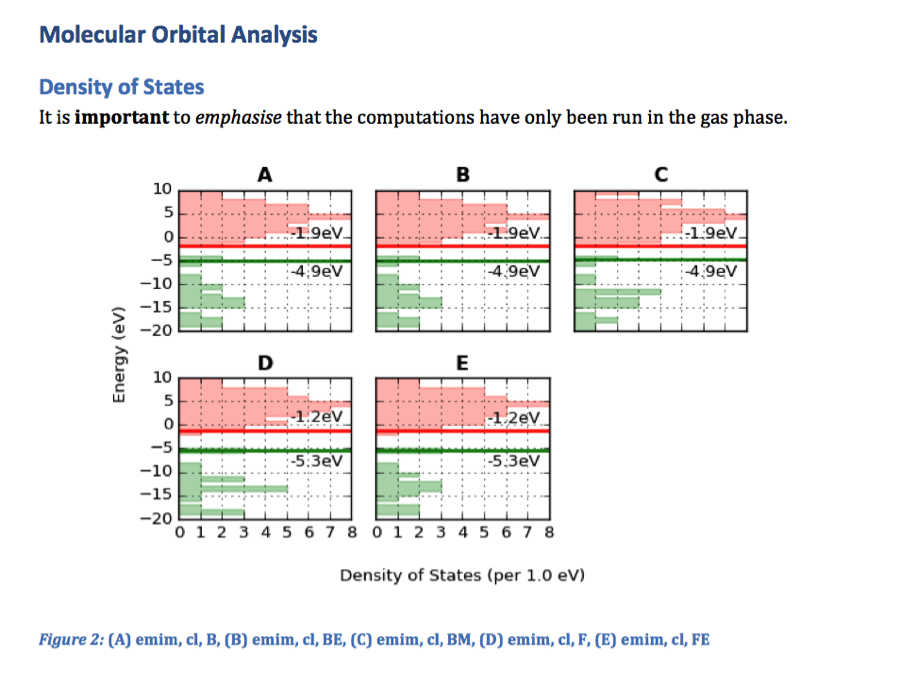
d.add_docstring("""
# Molecular Orbital Analysis
## Density of States
It is **important** to *emphasise* that the
computations have only been run in the gas phase.
""")
fig, caption = analysis.plot_mol_graphs(gtype='dos', max_cols=3,
lbound=-20, ubound=10, legend_size=0,
band_gap_value=False,
info_columns=['Cation', 'Anion', 'Initial'])
d.add_mpl(fig, dpi=96, height=9, caption=caption)
plt.close()
d.save('exmpl_assess.docx')
Which gives us the following:
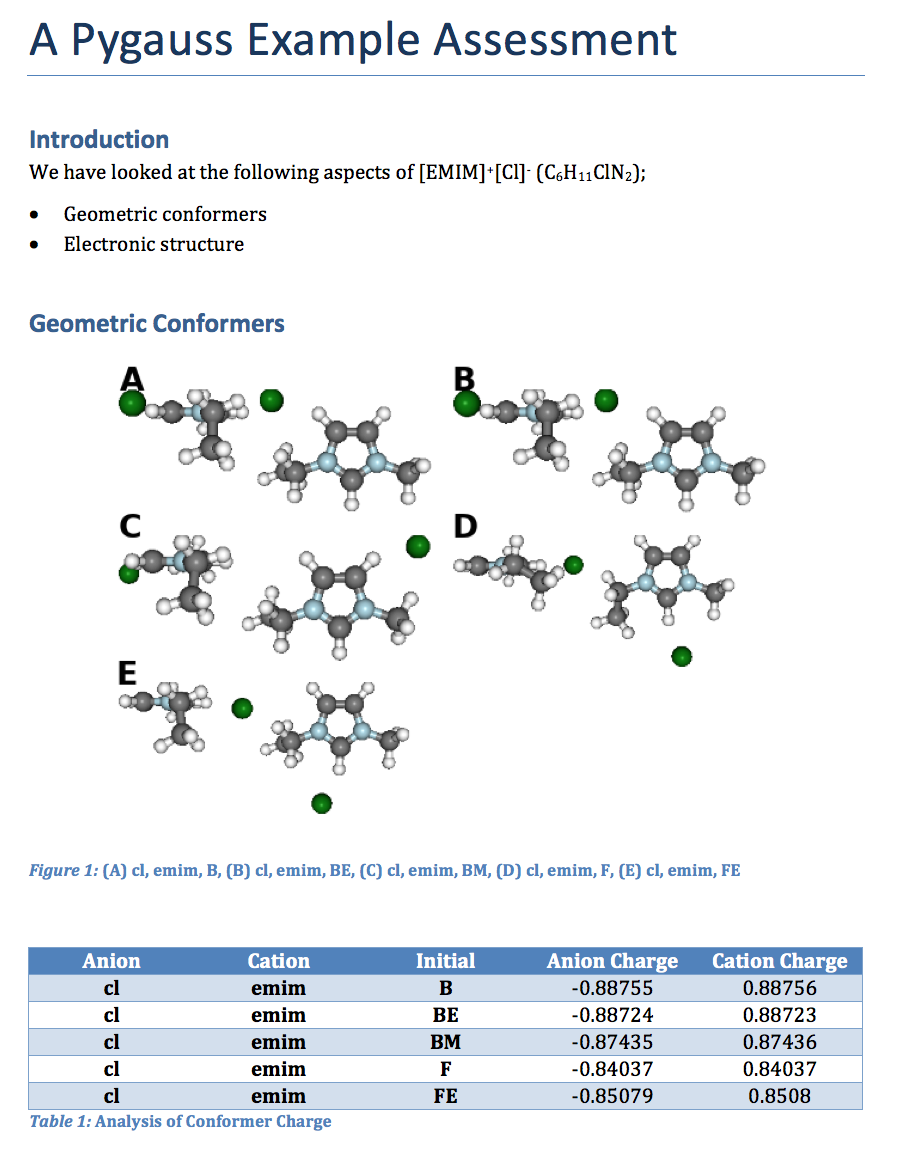
Further Examples¶
For more complex examples, you can also find a notebook from a recent project at; https://github.com/chrisjsewell/PyGauss/blob/master/Real_project_example_notebook.ipynb