Example Assessment¶
You should be able to open an IPython Notebook and perform the the following:
from IPython.display import display
%matplotlib inline
import pygauss as pg
print 'pygauss version: {}'.format(pg.__version__)
pygauss version: 0.4.0
and access the test folder with a number of example Gaussian outputs.
folder = pg.get_test_folder()
len(folder.list_files())
33
Note: the folder object will act identical whether using a local path or one on a server over ssh (using paramiko):
folder = pg.Folder('/path/to/folder',
ssh_server='login.server.com',
ssh_username='username')
Single Molecule Analysis¶
A molecule can be created containg data about the inital geometry, optimisation process and analysis of the final configuration. Molecules can be viewed statically or interactively (not currently supported by Firefox).
mol = pg.molecule.Molecule(folder_obj=folder,
init_fname='CJS1_emim-cl_B_init.com',
opt_fname=['CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_opt-modredundant_difrz.log',
'CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_opt-modredundant_difrz_err.log',
'CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_opt-modredundant_unfrz.log'],
freq_fname='CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_freq_unfrz.log',
nbo_fname='CJS1_emim-cl_B_6-311+g-d-p-_gd3bj_pop-nbo-full-_unfrz.log',
atom_groups={'emim':range(20), 'cl':[20]},
alignto=[3,2,1])
#mol.show_initial(active=True)
display(mol.show_initial(represent='vdw', rotations=[[0,0,90], [-90, 90, 0]]))
display(mol.show_optimisation(represent='ball_stick', rotations=[[0,0,90], [-90, 90, 0]]))
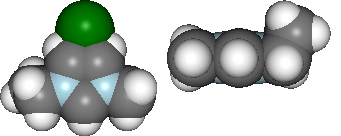
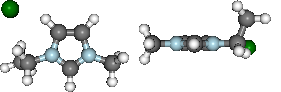
Basic analysis of optimisation...
print('Optimised? {0}, Conformer? {1}, Energy = {2} a.u.'.format(
mol.is_optimised(), mol.is_conformer(),
round(mol.get_optimisation_E(units='hartree'),3)))
ax = mol.plot_optimisation_E(units='hartree')
ax.get_figure().set_size_inches(3, 2)
ax = mol.plot_freq_analysis()
ax.get_figure().set_size_inches(4, 2)
Optimised? True, Conformer? True, Energy = -805.105 a.u.
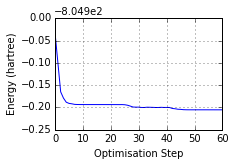

Geometric analysis...
print 'Cl optimised polar coords from aromatic ring : ({0}, {1},{2})'.format(
*[round(i, 2) for i in mol.calc_polar_coords_from_plane(20,3,2,1)])
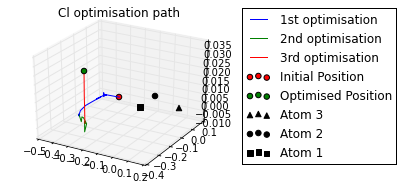
ax = mol.plot_opt_trajectory(20, [3,2,1])
ax.set_title('Cl optimisation path')
ax.get_figure().set_size_inches(4, 3)
Cl optimised polar coords from aromatic ring : (0.11, -116.42,-170.06)
Potential Energy Scan analysis of geometric conformers...
mol2 = pg.molecule.Molecule(folder_obj=folder, alignto=[3,2,1],
pes_fname=['CJS_emim_6311_plus_d3_scan.log',
'CJS_emim_6311_plus_d3_scan_bck.log'])
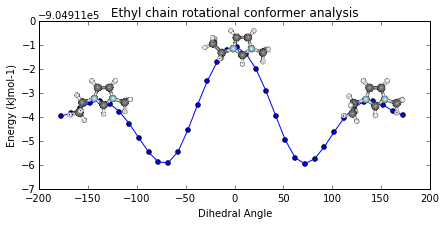
ax = mol2.plot_pes_scans([1,4,9,10], rotation=[0,0,90], img_pos='local_maxs', zoom=0.5)
ax.set_title('Ethyl chain rotational conformer analysis')
ax.get_figure().set_size_inches(7, 3)
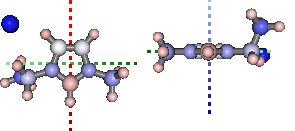
Natural Bond Orbital and Second Order Perturbation Theory analysis...
print '+ve charge centre polar coords from aromatic ring: ({0} {1},{2})'.format(
*[round(i, 2) for i in mol.calc_nbo_charge_center(3, 2, 1)])
display(mol.show_nbo_charges(represent='ball_stick', axis_length=0.4,
rotations=[[0,0,90], [-90, 90, 0]]))
+ve charge centre polar coords from aromatic ring: (0.02 -51.77,-33.15)
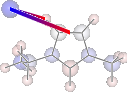
print 'H inter-bond energy = {} kJmol-1'.format(
mol.calc_hbond_energy(eunits='kJmol-1', atom_groups=['emim', 'cl']))
print 'Other inter-bond energy = {} kJmol-1'.format(
mol.calc_sopt_energy(eunits='kJmol-1', no_hbonds=True, atom_groups=['emim', 'cl']))
display(mol.show_sopt_bonds(min_energy=1, eunits='kJmol-1',
atom_groups=['emim', 'cl'],
no_hbonds=True,
rotations=[[0, 0, 90]]))
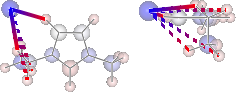
display(mol.show_hbond_analysis(cutoff_energy=5.,alpha=0.6,
atom_groups=['emim', 'cl'],
rotations=[[0, 0, 90], [90, 0, 0]]))
H inter-bond energy = 111.7128 kJmol-1
Other inter-bond energy = 11.00392 kJmol-1
Multiple Computations Analysis¶
Multiple computations, for instance of different starting conformations, can be grouped into an Analysis class.
analysis = pg.Analysis(folder_obj=folder)
errors = analysis.add_runs(headers=['Cation', 'Anion', 'Initial'],
values=[['emim'], ['cl'],
['B', 'BE', 'BM', 'F', 'FE']],
init_pattern='*{0}-{1}_{2}_init.com',
opt_pattern='*{0}-{1}_{2}_6-311+g-d-p-_gd3bj_opt*unfrz.log',
freq_pattern='*{0}-{1}_{2}_6-311+g-d-p-_gd3bj_freq*.log',
nbo_pattern='*{0}-{1}_{2}_6-311+g-d-p-_gd3bj_pop-nbo-full-*.log',
alignto=[3,2,1], atom_groups={'emim':range(20), 'cl':[20]})
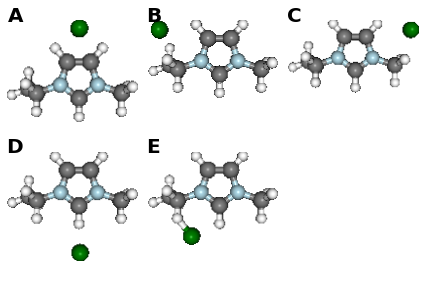
fig, caption = analysis.plot_mol_images(mtype='initial', max_cols=3,
info_columns=['Cation', 'Anion', 'Initial'],
rotations=[[0,0,90]])
print caption
Figure: (A) emim, cl, B, (B) emim, cl, BE, (C) emim, cl, BM, (D) emim, cl, F, (E) emim, cl, FE
The methods mentioned for indivdiual molecules can then be applied to all or a subset of these computations.
analysis.add_mol_property_subset('Opt', 'is_optimised', rows=[2,3])
analysis.add_mol_property('Energy (au)', 'get_optimisation_E', units='hartree')
analysis.add_mol_property('Cation chain, $\\psi$', 'calc_dihedral_angle', [1, 4, 9, 10])
analysis.add_mol_property('Cation Charge', 'calc_nbo_charge', 'emim')
analysis.add_mol_property('Anion Charge', 'calc_nbo_charge', 'cl')
analysis.add_mol_property(['Anion-Cation, $r$', 'Anion-Cation, $\\theta$', 'Anion-Cation, $\\phi$'],
'calc_polar_coords_from_plane', 3, 2, 1, 20)
analysis.add_mol_property('Anion-Cation h-bond', 'calc_hbond_energy',
eunits='kJmol-1', atom_groups=['emim', 'cl'])
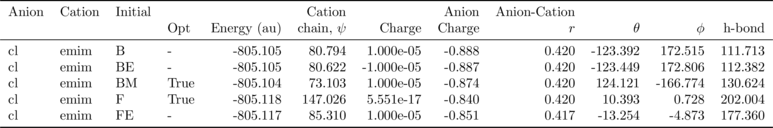
tbl = analysis.get_table(row_index=['Anion', 'Cation', 'Initial'],
column_index=['Cation', 'Anion', 'Anion-Cation'])
NEW FEATURE: there is now an option (requiring pdflatex and ghostscript+imagemagik) to output the tables as a latex formatted image.
analysis.get_table(row_index=['Anion', 'Cation', 'Initial'],
column_index=['Cation', 'Anion', 'Anion-Cation'],
as_image=True, font_size=12)
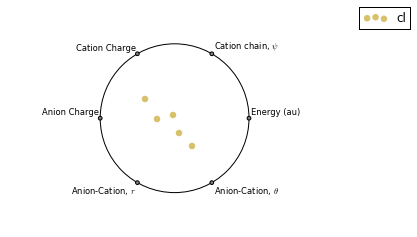
RadViz is a way of visualizing multi-variate data.
ax = analysis.plot_radviz_comparison('Anion', columns=range(4, 10))
The KMeans algorithm clusters data by trying to separate samples into n groups of equal variance.
pg.utils.imgplot_kmean_groups(
analysis, 'Anion', 'cl', 4, range(4, 10),
output=['Initial'], mtype='optimised',
rotations=[[0, 0, 90], [-90, 90, 0]],
axis_length=0.3)
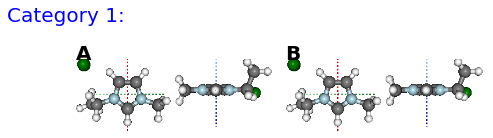
Figure: (A) B, (B) BE
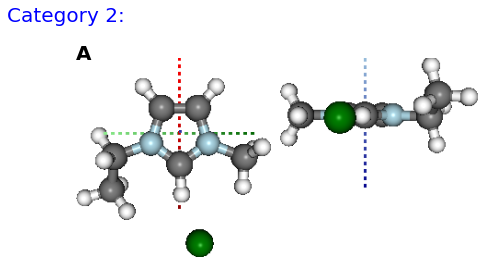
Figure: (A) BM
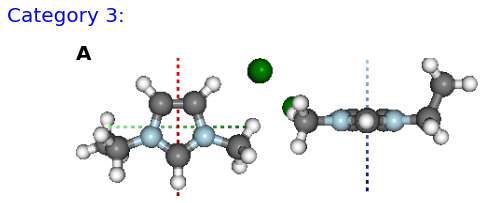
Figure: (A) FE
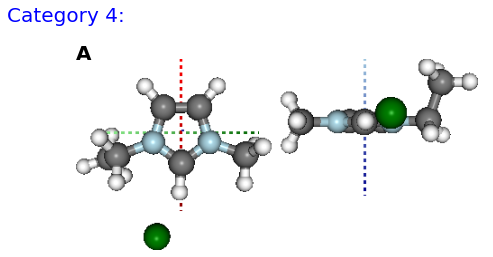
Figure: (A) F
MORE TO COME!!